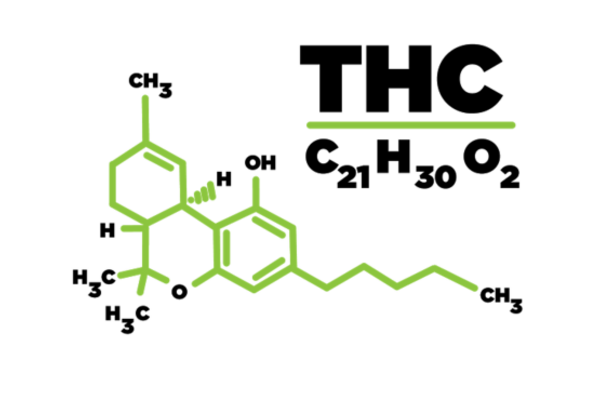
The Advisory Council on the Misuse of Drugs (ACMD) published an assessment of the impact of rescheduling Cannabis Based Products for Medicinal use (CBPMs) to Schedule 2 under the Misuse of Drugs Regulations 2001 (MDR) and recommendations to mitigate the issues identified.
ACMD - Cannabis Based Products for Medicinal use
On the 11th January, Kit Malthouse wrote to the chair of the ACMD asking:
"that the defined trace percentage in CBD products be set at a level which will be between 0.01% and 0.0001% by weight per controlled cannabinoid.
"that the ACMD considers the maximum dose for any non-negligible effect for THCV, Δ9-THC and CBN and the cannabinoid Δ9THCA-A. We are seeking further scientific testing advice on analytical capability to test for a cannabinoid content of 0.01-0.0001% by weight or lower per specified cannabinoid. In particular, we would ask that the ACMD considers whether such products would be liable to be abused or have ill effects, and whether the controlled substances could, in practice, be recovered from such products."