‘Aches & Pains’ and ‘Inflammation’ are bandied around with no consequence when these are medicinal claims and used by advertising agencies and new start-ups who are unaware of the possible outcomes and penalties that entire industry could suffer as a result.
In 2016, MHRA published an opinion that products containing CBD, when used for a medical purpose, should be considered medicinal. They urged voluntary compliance from companies providing the product and ‘Guidance Note 8 – A guide to what is a medicinal product’ was published to provide supplemental information about regulations surrounding sales of CBD for non-medicinal purposes.
Any person or company marketing a product has a responsibility to do so in accordance with the law. The Regulations provide that, unless exempt, any medicinal product placed on the UK market must have a marketing authorisation (MA), traditional herbal registration (THR) or certificate of registration as a homoeopathic product granted by the European Commission or by the UK Licensing Authority.
It is an offence to sell or supply or to advertise a medicinal product which does not have authorisation.
Is my product medicinal?
Ask yourself – does this product have a licence to be medicinal?
We know that cannabis is a medicinal herb, but has your company licensed your product to be recognised as a medicine?
What is a Medicinal Product
A "medicinal product" is defined in Article 1 of Directive 2001/83/EC, which is implemented by Regulation 2 of the Regulations. The definition, which is in two ‘limbs’ is:
- Any substance or combination of substances presented as having properties for treating or preventing disease in human beings (Limb 1).
- Any substance or combination of substances which may be used in or administered to human beings either with a view to restoring, correcting, or modifying physiological functions by exerting a pharmacological, immunological, or metabolic action, or to making a medical diagnosis (Limb 2).
If a product satisfies either of the above criteria, it may be classed as a medicinal product. In broad terms, when classifying a product, the Agency looks at the way it is presented and its function, that is, its effects (when administered) on human physiology.
What is a Disease
The term “disease” is defined in Regulation 8 of the Regulations as:
“includes any injury, ailment or adverse condition, whether of body or mind.”
Clinical studies have shown that CBD has a clear and demonstrable therapeutic effect, especially for people suffering from severe epilepsy. Furthermore, MHRA's clinical assessments justify the mode of action of CBD when it comes to its effectiveness in treating various medical conditions such as graft-versus-host disease, perinatal asphyxia, and Dravet Syndrome.
Any product that makes a medicinal claim would fall within Limb 1 of the definition. For the avoidance of doubt, this includes any testimonies that are included on websites or any other promotional material. Further guidance in relation to medicinal claims can be found in Appendices 1, 8 and 9 of Guidance Note 8.
What is a Medicinal Claim
When considering borderline products, the MHRA considers the following examples to be medicinal claims:
- references to all medical conditions major to minor including colds, headaches, cuts and bruises, smoking addiction, obesity, arthritis, depression, stress and all childhood disorders and serious diseases.
- references to condition of the mind such as depression, PTSD, addictions, attention deficit/hyperactivity disorder.
- references to treatment or alleviation of adverse conditions including decongests, relieves pain, reduces inflammation, calms, stops itching, cures insomnia, reduces blood pressure, reduces sugar levels.
- references to the symptoms of disease or injury, such as pain/aches, inflammation, rashes etc.
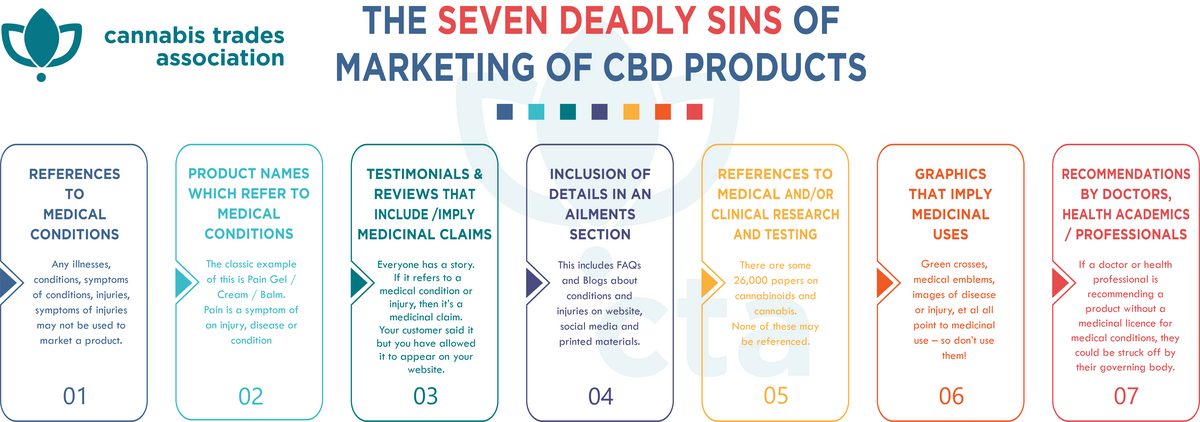
Improper marketing of CBD Products
Forms of marketing MHRA consider may suggest to a consumer that a product is properly classified as a medicinal product:
- references to medical conditions
- comparison with licensed medicines
- references to interference with the normal operation of a physiological function
- product names which refer to adverse medical conditions
- references to medical and / or clinical research and testing
- references to the health risks of not taking a particular product
- editorial medicinal claims
- recommendations by Doctors/health professionals
- testimonials from customers that include/imply medicinal claims
- graphics that imply medicinal uses
- references to or reproduction of "generic" information
- juxtaposing with any examples of the above
- inclusion of details in an Ailments Section.
Claims to “maintain” health
The MHRA view is that claims to “maintain” or “help to maintain” “dietary maintenance” or “support” health or a healthy lifestyle, can be approved under food law, and would not normally regard such claims to be medicinal. Nor, if such claims are clearly made in relation to healthy bodily functions or organs, is the MHRA likely to consider them as presenting the product for treating disease.
In general, the MHRA is only likely to consider “health maintenance” claims as medicinal if they suggest or imply that a product may prevent disease or, where targeted on a vulnerable section of the population, may restore, or help to restore, a specific bodily function or organ to a normal healthy state.
Compliance with other regulatory frameworks.
MHRA will never advise that a product is ‘legal.’ This is because their remit is limited to the regulation of medicines and medical devices and any advice given in relation to CBD products will be in respect of classifying as a medicinal product.
Products which are not deemed to be medicine will be subject to other regulatory frameworks and it is the responsibility of those who manufacture and/or market a product to ensure that they comply with the relevant legislation.
The MHRA encourages companies to seek information from appropriate authorities.
Novel Foods Authorisation.
When consumed as food, CBD extracts, isolates, distillates, and other new forms of CBD are novel foods.
Hemp products – including cold-pressed oils - are not considered to be novel (or out of historical context) because there is evidence to show that hemp was eaten before May 1997. However, this is not the case for CBD extracts i.e., concentrated forms derived from hemp/cannabis.
The Endocannabinoid System (ECS)
MHRA is sometimes questioned about whether claims can be made for CBD regarding the ECS. The endocannabinoid system is a neuromodulator system that regulates physical and mental functions.
Claims which imply that CBD might change, stimulate, or enhance the ECS (or similar) may in context, be considered medical claims.
If such claims are not medically related, companies should consider food regulations when making these statements concerning this supplement; otherwise contact local authorities such as Trading Standards or Environmental Health to see if they need permission before publishing these statements on websites or social media platforms.
Cosmetic claims
Cosmetic claims should emphasise the cosmetic use of the product i.e., cleansing, moisturising, perfumery, keeping the skin in good condition. As a guide the following are examples of claims which MHRA could regard to be adverse medical conditions:
- To protect /prevent eczema, dermatitis, and psoriasis.
These are all adverse medical conditions which can be exhibited by dry, inflamed, scaly and itchy skin and products will fall to be either medicinal products or medical devices depending on their mode of action.
MHRA has also agreed with the CTPA that consumers with an existing adverse skin condition may wish to know if a cosmetic product is appropriate for them to use. Where this can be substantiated, the following wording can be used.
- “Also suitable for people who may be prone to eczema / psoriasis / dermatitis /rosacea / acne / spots.”
This wording should be present for advisory purposes and should not be used in a manner which could imply, either explicitly or implicitly, that they can be used for the prevention or treatment of these conditions.
- Products to treat/prevent or fight spots and acne are medicines
These are all adverse medical conditions. Cosmetic products may also claim ‘also suitable for people who may be prone to acne/spots’ if claims do not give the impression, explicitly or implicitly, that they can be used for the prevention or treatment of acne or other adverse skin conditions.
Medical Devices
Products that incorporate, or are used to administer, a drug may be regulated as either medical devices or as medicinal products, depending on the principal intended function of the product and the method by which this action is achieved.
The principle mode of action for a medical device is typically fulfilled by physical means (including mechanical action, physical barrier, replacement of, or support to, organs or body functions).
Medical devices may contain medicinal substances, including herbal and plant extracts and substances derived from human blood or blood plasma, which act on the body in a manner ancillary to the device. However, when such substances act in a manner that is more than ancillary, the product is likely to be regulated as a medicinal product.
CBD products for medical purposes.
In the absence of any licensed CBD medicinal products, MHRA would advise individuals with underlying medical conditions who are using CBD to discuss this with their doctor who may prescribe medicinal products to appropriately manage the symptoms. Companies wishing to supply CBD products for a medical purpose should consider the following:
1. Use of an unlicensed medicine (Special).
Unregulated medicines containing cannabis-derived substances can be prescribed for treatment under some circumstances, but there are limitations. Medicine must have a license before being placed on the market, but unregulated medicinal products with cannabis-derived substances (CBD) can be made available to patients based on prescription. Unregulated medicines containing CBD could only be given out if it meets the individual needs of that patient. Prescribing under these circumstances should belong strictly to the physician in charge and will depend solely on what he/she sees fit based upon his/her professional judgement. These unregulated medicines cannot be advertised or marketed; a manufacturer, distributor or wholesaler may provide factual answers about their product without making medical claims though.
2. Obtaining a license to sell a licensed medicine.
Where CBD is being considered for a clinical trial in humans (a regulatory agency responsibility), the material needs to be manufactured or imported by the holder of an appropriate company authorisation from manufacturing an investigational drug, and it needs to have been approved for research use.
The MHRA’s Guidance Note 8 document outlines its current stance on the classification of cannabidiol (CBD) products. It will also cover how suppliers may provide these products as medicinal remedies. Before supplying any product for sale, it is vital for businesses to consult with all applicable regulatory bodies - otherwise there could be legal repercussions.