
The enforcement of the Act falls under the Advertising Standards Authority (ASA) and the MHRA, which possess the authority to investigate and take legal action against individuals or companies who violate its provisions.
The Act covers all forms of advertising, including online and print ads, radio and TV broadcasts, and public statements made by healthcare professionals or advocates. It also applies to any product or service that claims to cure or treat cancer, regardless of its source or composition.
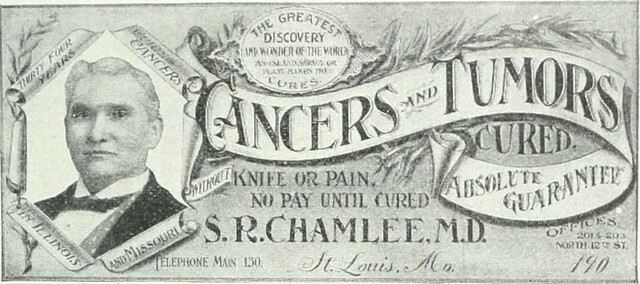
While some have criticised the Act for restricting access to alternative or complementary cancer treatments, supporters believe it plays an essential role in safeguarding patients from fraudulent or hazardous treatments that may do more harm than good. The aim of the legislation is to safeguard patients from unscrupulous individuals and vendors who sell bogus remedies, which posed a genuine threat to cancer sufferers in the past and continue to do so today. The limited number of legal actions taken under the Act is in line with the original intention of the legislation. In recent times, there have been demands to update the Act to align with changes in healthcare technology and practices. However, it remains a crucial component of the UK's legal framework for cancer prevention and treatment.

It's important for business owners selling CBD and cannabis products to be familiar with the Cancer Act. The Act is overseen by the Advertising Standards Authority (ASA) and the Medicines and Healthcare products Regulatory Agency (MHRA), which have the authority to investigate and take legal action against those who violate its provisions. Therefore, it's essential that CBD or cannabis businesses advertising products ensure that their marketing materials don't contain false, misleading, or inaccurate claims inferring the cancer-curing abilities of their products. Failure to comply with this requirement could result in legal consequences, such as fines and other penalties.
Furthermore, even if a CBD or cannabis product has genuine therapeutic properties, it must adhere to the regulations established by the MHRA prior to making any health claims. This entails acquiring the necessary licenses and authorizations, as well as conducting clinical trials and providing scientific proof to support any health-related assertions.
To summarize, businesses marketing CBD and cannabis products must be mindful of the Cancer Act of 1939 and the accompanying regulations to avoid violating any provisions, which may result in legal repercussions.





